Our Institute
Clinical Trials
Our Science
News
International Patients
- International Patients
- International patient Service Care
- Travel Arrangement and Hospital Admission
- FAQ
- Contact Us
Cancer History:
First diagnosis of cancer: 12/2018 Primary disease: B-ALL
First recurrence date: 06/2019 Relapsed disease sites: bone marrow
Therapy gone through: Induction and maintenance chemotherapy
Surgery: No
Chemotherapy: 5 cycles, IC BFM 2009/ Augmented Induction B(incomplete)/ HAM/FLAG IDA/ RE INDUCTION IA regimen;
Radiotherapy: No
Targeted drugs: No Other immunotherapy: No Others: Tapering of Prednisone 10 mg per day
Clinical Trial Number: NCT04016129 ; enrollment date: 28/10/2019
Interventions:
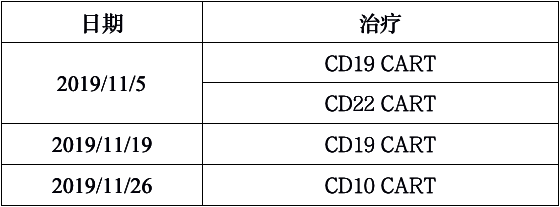

4SCAR (CD19, CD22,CD10)after Flu-Cy
CRS Grade: III ;
other side effects: lung infection
Treatment response: CR
Quality of Life: improved
Progression-free interval: from Dec 2019 till now
Current status: CR, HSCT is recommended