31岁原发性纵隔b细胞淋巴瘤男性患者,扩散在胸内、心包淋巴结…
患者:男,31岁,原发性纵隔b细胞淋巴瘤
主要症状:累及胸内、心包淋巴结
首次确诊:2016年8月
治疗经历 手术:是 2017年5月肺部
化疗:是 6个周期R-DA-EPOCH,1个周期R-DHAP,1个周期dexaBEAM,1个周期IGEV+oxaliplatin
免疫治疗:是 2个周期纳武单抗,来那度胺
其他:否
临床试验编号:NCT03125577 入组治疗日期:2017年6月28日
方案:4SCART (CD19, CD30)
CRS反应级别:0级 其他副作用:无
疗效判断:CR(完全缓解,纳武单抗+来那度胺之后)
生活质量:提高
疾病无进展时间间隔:1年
目前状况:PMBCL转化为HL,治疗有效
CART治疗前后CT影像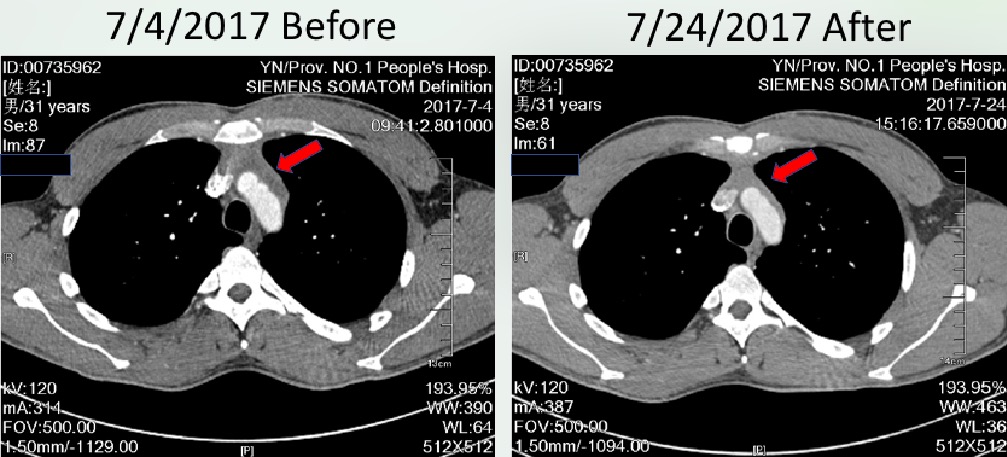
发表论文链接:
1)Chang LJ, Dong LJ, Zhu J. et al. (2015) 4SCAR19 Chimeric Antigen Receptor-Modified T Cells As a Breakthrough Therapy for Highly Chemotherapy-Resistant Late-Stage B Cell Lymphoma Patients with Bulky Tumor Mass. Blood 126 (23), 264.
2)Jing HM, Chang LJ, Chen MY, Bao F, Wang J, Wang WM, Liu YY, Kuo HH, Liu YC, Dong LJ. (2015) Up-Regulation of CAR-T Cells after G-CSF and Dexamethasone Treatment in a Defuse Large B Cell Lymphoma Patient Receiving 4SCAR19 T Cell Therapy. Blood 126 (23), 5130.
3)L-J Chang, Lujia D, Liu Y-C, Tsao S-T, Li Y-C, Liu L, Gao Z, Tan X, Lu D-P, Zhang J-P, Wang J-B, Ying Y-M, Zhang L-P, Zheng H, Wang K, Zheng X-L, Wang H-X, Lai X, and Li D (2016) Safety and Efficacy Evaluation of 4SCAR19 Chimeric Antigen Receptor-Modified T Cells Targeting B Cell Acute Lymphoblastic Leukemia - Three-Year Follow-up of a Multicenter Phase I/II Study. Blood 128 (22), 587.
4) Hao L, Li T, Chang L-J and Chen X. (2017) Adoptive immunotherapy for B-cell malignancies using CD19-targeted chimeric antigen receptor T cells: a systematic review of efficacy and safety. Curr Med Chem.
5)Chang, L.-J., Li, Y. Tu, S. et al. Phase I/II Trial of Multi-Target Chimeric Antigen Receptor Modified T Cells (4SCAR2.0) Against Relapsed or Refractory Lymphomas. (2018) Blood 132:225.
患者反馈信息:待发