![]()
Chimeric Antigen Receptor T-Cells (CAR-T) Therapy: a living drug
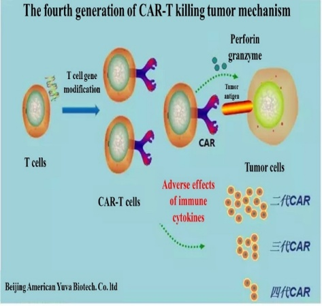
Chimeric Antigen Receptor (CAR)-T cell therapy is a treatment based on gene therapy technology to modify T cells.
There are three key elements to this technology:
1.A genetically modified T cell receptor based on B cell antibody gene and T cell receptor gene.
2.A gene transfer tool that can effectively modify T cells.
3.The modified T cells that boost the immune system and helps to maintain the good function inside the body.
CAR-T cell therapy is a cancer targeting live drug in which the CAR directs the modified T cells to target and kill cancer. CAR-T can target a cancer cell by binding to specific cancer cell surface antigen. Many cancer cell surface antigens have been tested for CAR-T cell therapy.The most important factor to the success of CAR-T cell therapy is the efficiency of gene transfer into T cells. Most CAR-T cell technologies use lentivirus vector as gene transfer tool because lentivector can efficiently enter T cells and maintain a prolonged gene expression.
The most popular CAR-T design has been the CD19-specific CAR. The 3rd generation CAR uses the T cell co-stimulatory factor 4-1BB signal to activate T cell, which can promote the proliferation of T cells and maintain a prolonged therapeutic effect. Although effective, 4-1BB-based CD19-CAR-T cell treatment is known to cause severe reactions including neutropenia, cytokine release syndrome (CRS), tumor lysis syndrome, fever, hypotension, and sometime neuropathy. While in one hand, CAR-T can rapidly and robustly kills cancer cells, it can also trigger a strong CRS that could be lethal. Many CD19-CAR-T cell treated leukemia patients enter intensive care unit (ICU) after developing severe CRS.
GIMI laboratory uses an advanced 4th generation CAR design (4SCAR), which has a unique feature: low or no CRS. After years of clinical trials, CD19-specific 4SCAR-T has demonstrated cancer remission rate similar to the 3rd generation CAR but without severe side effects!
GIMI’s 4th generation CAR-T technology has treated more than 1000 patients, including leukemia, lymphoma and solid tumor patients, illustrating high safety and efficacy. These studies have been published annually since 2013 in international conferences including ASH, AACR, ASCO and ASGCT.
Cytokine releasing syndrome (CRS) is the most common side effect of CAR-T therapy. Symptoms of CRS include high fever, low blood pressure and neurological disorders.
GIMI's fourth-generation CAR (4SCAR) not only has a significant improvement in efficacy, but most importantly, 4SCAR has high safety feature by integrating multiple co-stimulatory factors and an inducible suicide gene.
Based on the 4th generation CAR-T design, we have developed more than 100 CAR-T target schemes, which target the vast majority of cancer types, and many have been applied to patients.
In 2015, we reported a 3-year follow-up of the 4SCAR19 trial in the annual meeting of the American Society of Hematology (ASH). The phase I/II study included more than 200 patients with leukemia and more than 30 patients with stage III/IV advanced lymphomas. After 4SCAR19 treatment, the prognosis of these patients was significantly improved, and the response rate of acute lymphoblastic leukemia patients was up to 94%, and more than 80% achieved complete remission (CR). The 4SCAR technology is internationally well-published.
Since 2015, we have advanced the CAR-T technology into the CAR-T 2.0 era. This advancement consists in a modified treatment scheme to include additional CAR-T infusions to target more than one target antigen in the tumor cells, and also, to extend the CAR-T effect in patients. In 2018, we reported an interim report of 4S19CAR2.0 study for the treatment of B cell lymphomas. The results illustrated significant improvement in CAR-T overall response rate and CR rate for patients receiving the CAR-T 2.0 treatment. Similar CAR-T 2.0 strategy has been applied to all cancers.
