![]() CAR-T CELLS THERAPY
CAR-T CELLS THERAPY
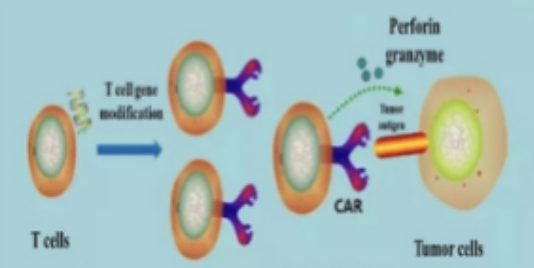
GIMI uses patented advanced 4th generation CAR design (4SCAR) with high efficacy and minimal side-effect (cytokine releasing syndrome – CRS). For many years GIMI has developed multiple different target CARs for blood cancer and solid tumors.
EIE THERAPY
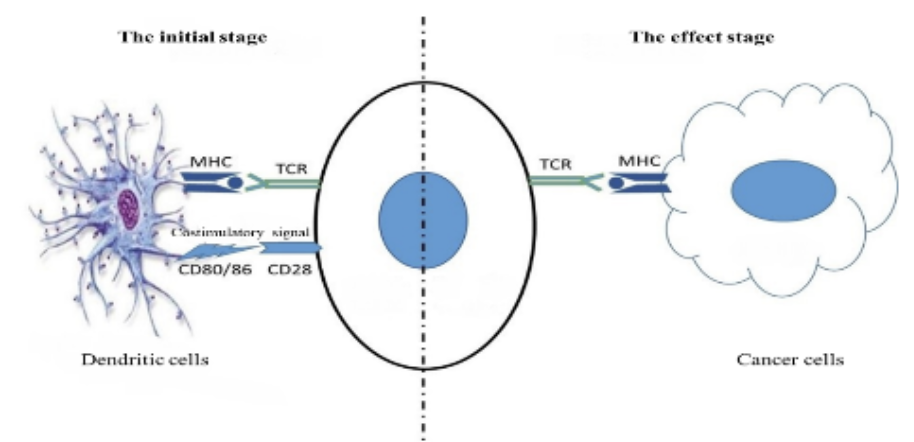
Engineered Immune Effector (EIE) therapy is tailor-made targeted immune cells for each patient. EIE recognizes multiple specific tumor-associated antigens and then exerts immune effector functions to kill tumor cells or pathogens (targeting virus and fungal infections, etc.).
Dendritic Cell (DC) Vaccines
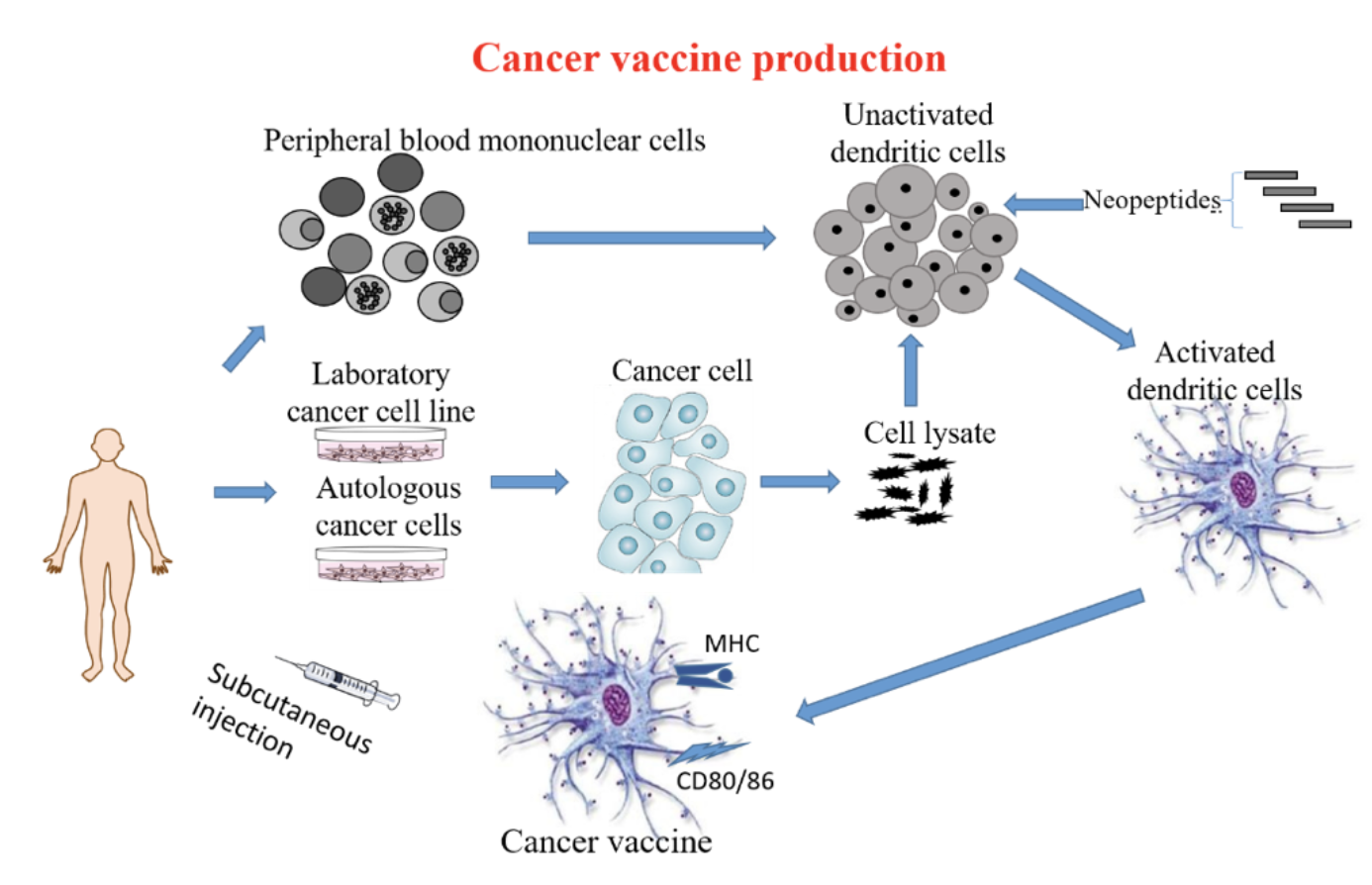
Using the latest technology (NGS WES and computer-aided HLA restricted epitope prediction technology) including immune gene-modified tumors to modify Dendritic Cells (DC) to produce immune gene-modified DC vaccines ready to be administered in patients.
Neoantigens or Neoepitopes
Neoantigens (Neoepidopes) are cancer-specific antigens based on the mutations particular to a cancer by using advanced sequencing technology to discover the difference between the DNA of a tumor and a patient’s normal cells. This is one of the newest tools in cancer immunotherapy. Because neoantigens target only the tumor, they tend to have less non-specific side effect. Neoantigens can be used in EIE and DC therapies.
GIMI´s portfolio offers powerful advantages to patients
GIMI’s immune portfolio options amplify the power of traditional immunotherapy by engaging adaptive and innate immune systems, directly and indirectly, with multi-target gene-associated anti-cancer immune strategies.
The broad CAR-T cell antigen portfolio allows GIMI to personalize therapy to patients’ needs with high safety and precise therapy.
The sequential multi-target 4SCARs and DC vaccines offers a distinctly powerful therapeutic strategy with high safety profile which are financially feasible and accessible to many patient.
Challenges for CAR-T cells in solid tumors and Acute Myeloid Leukemia
The two main challenges that CAR/TCR T-cell therapies face in the treatment of solid tumors are the heterogenous expression and escape of the CAR/TCR target antigens and local immunosuppression that impedes their clinical implementation:
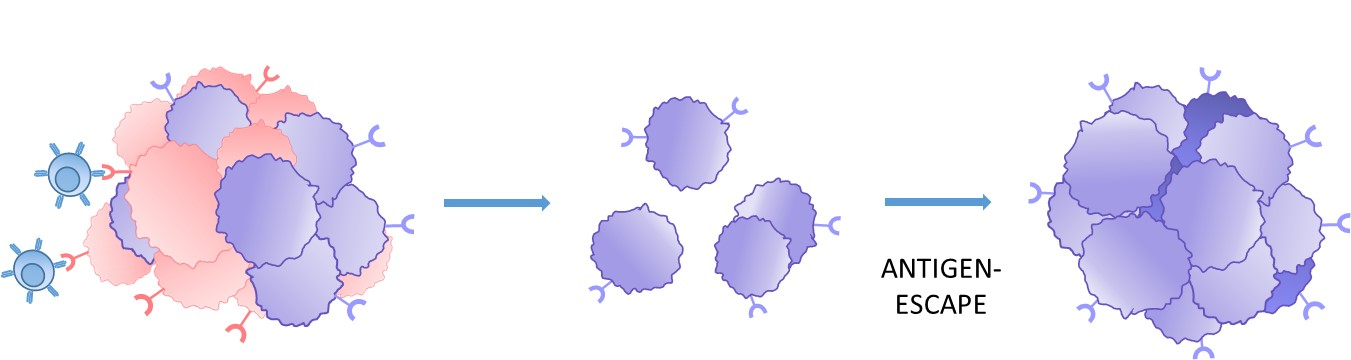 1. Tumor target antigen heterogeneity
1. Tumor target antigen heterogeneity
Heterogenous antigen expression or loss on tumor cells increases the risk of antigen-escape and the formation of CAR-T resistant tumors.
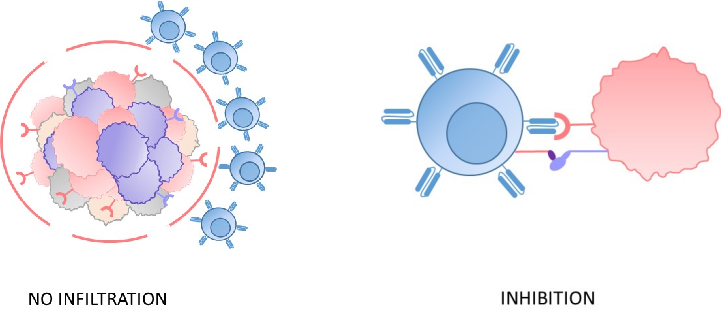
2. Immunosuppressive tumor microenvironment
The microenvironment of solid tumors and AML is highly immunosuppressive, which exhausts the CAR-T cells and makes it more difficult for them to infiltrate tumors, thus limiting their potential to kill cancer cells.
Multi-antigen targets - GIMI´s technology
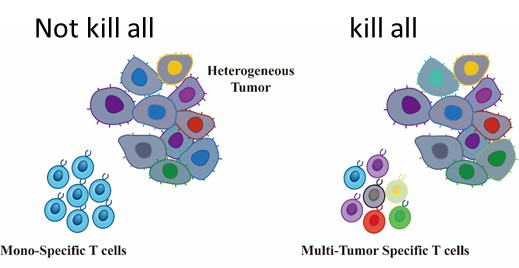 GIMI´s technology utilizes a multi-antigen CAR-T cell therapy approach accordingly to tumor gene expression. The multi-antigen approach has been well-tolerated and shown to enhance tumor-killing capability with prolonged efficacy.
GIMI´s technology utilizes a multi-antigen CAR-T cell therapy approach accordingly to tumor gene expression. The multi-antigen approach has been well-tolerated and shown to enhance tumor-killing capability with prolonged efficacy.
Choosing the right therapy
Selecting the right therapy is on a case-by-case basis. Generally, patients with a compromised immune system as a result of cancer or chemotherapy will benefit more from CAR-T and EIE and less from DC. This is because CAR-T and EIE technologies can expand the patient’s immune cells in culture and greatly increase them before infusion back to the patient.
Patients with an intact immune system can benefit from all therapies, with DC being the easiest and cheapest treatment with few side effects.
The choice of immune cell treatment is also based on testing results, and the best options may depend on the type of target antigens and the individual patient’s condition.
Testing for the right antigen target
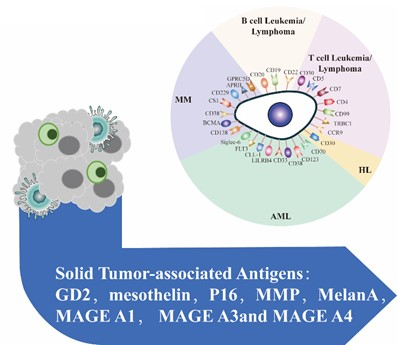
In order to design the best cell therapy against the patient’s specific tumor, tumor testing must first be performed unless the target antigens are well known. Our service comprehensively analyzes antigen repertoire using blood and tissue samples' most advanced technology (Flow Cytometry [FACS], Immunohistochemistry [IHC] staining, next-generation sequencing [NGS], and RT-pPCR/ddPCR). A detailed data analysis will be performed to study which antigens are more suitable for immune therapy. We ensure that we consistently perform this service, from sample preparation to delivering results.
Manufacturing

GIMI’s manufacturing process is the school of immune cells. The first step is to harvest cells from patients' blood and separate the cells required for each therapy.
CAR-T therapy – 1 week of preparation time.
EIE therapy – 20 days of preparation time.
DC therapy – 5 days of preparation time.
All types of Adoptive Cell Therapy require an initial blood cell draw, called apheresis. This can be done in half a day. The patient can stay in hospital during this preparation or return home when the cells are ready to be administered. In solid tumors, the sample is processed to analyze antigens presence and/or processed to obtain antigens peptides to produce EIE or DC vaccine.


 Engineered Immune Effector (EIE) therapy is tailor-made targeted immune cells for each patient. EIE recognizes multiple specific tumor-associated antigens and then exerts immune effector functions to kill tumor cells or pathogens (targeting virus and fungal infections, etc.).
Engineered Immune Effector (EIE) therapy is tailor-made targeted immune cells for each patient. EIE recognizes multiple specific tumor-associated antigens and then exerts immune effector functions to kill tumor cells or pathogens (targeting virus and fungal infections, etc.). 
 Using the latest technology (NGS WES and computer-aided HLA restricted epitope prediction technology) including immune gene-modified tumors to modify Dendritic Cells (DC) to produce immune gene-modified DC vaccines ready to be administered in patients.
Using the latest technology (NGS WES and computer-aided HLA restricted epitope prediction technology) including immune gene-modified tumors to modify Dendritic Cells (DC) to produce immune gene-modified DC vaccines ready to be administered in patients.
 GIMI’s immune portfolio options amplify the power of traditional immunotherapy by engaging adaptive and innate immune systems, directly and indirectly, with multi-target gene-associated anti-cancer immune strategies.
GIMI’s immune portfolio options amplify the power of traditional immunotherapy by engaging adaptive and innate immune systems, directly and indirectly, with multi-target gene-associated anti-cancer immune strategies. 1. Tumor target antigen heterogeneity
1. Tumor target antigen heterogeneity



 GIMI´s technology utilizes a multi-antigen CAR-T cell therapy approach accordingly to tumor gene expression. The multi-antigen approach has been well-tolerated and shown to enhance tumor-killing capability with prolonged efficacy.
GIMI´s technology utilizes a multi-antigen CAR-T cell therapy approach accordingly to tumor gene expression. The multi-antigen approach has been well-tolerated and shown to enhance tumor-killing capability with prolonged efficacy.

 In order to design the best cell therapy against the patient’s specific tumor, tumor testing must first be performed unless the target antigens are well known. Our service comprehensively analyzes antigen repertoire using blood and tissue samples' most advanced technology (Flow Cytometry [FACS], Immunohistochemistry [IHC] staining, next-generation sequencing [NGS], and RT-pPCR/ddPCR). A detailed data analysis will be performed to study which antigens are more suitable for immune therapy. We ensure that we consistently perform this service, from sample preparation to delivering results.
In order to design the best cell therapy against the patient’s specific tumor, tumor testing must first be performed unless the target antigens are well known. Our service comprehensively analyzes antigen repertoire using blood and tissue samples' most advanced technology (Flow Cytometry [FACS], Immunohistochemistry [IHC] staining, next-generation sequencing [NGS], and RT-pPCR/ddPCR). A detailed data analysis will be performed to study which antigens are more suitable for immune therapy. We ensure that we consistently perform this service, from sample preparation to delivering results.
 GIMI’s manufacturing process is the school of immune cells. The first step is to harvest cells from patients' blood and separate the cells required for each therapy.
GIMI’s manufacturing process is the school of immune cells. The first step is to harvest cells from patients' blood and separate the cells required for each therapy.