Our Institute
Clinical Trials
Our Science
News
International Patients
- International Patients
- International patient Service Care
- Travel Arrangement and Hospital Admission
- FAQ
- Contact Us
A 75 year-old woman sufferred with mucosa-associated lymphoid tissue lymphoma (MALT) for many years…
Cancer History:
First diagnosis of cancer: 05/2005 Primary disease sites: MALT lymphoma
First recurrence date: 11/2012 Relapsed disease sites: the upper lobe of left lung
Therapy gone through:
Surgery: No
Chemotherapy: 7 cycles, CHOP regimen; radiotherapy: Yes, 30Gy (Stomach)
Targeted drugs: Rituximab other immunotherapy: No others: Thalidomide
Clinical Trial Number: NCT02247609 ; enrolled date: 16/09/2017
Interventions:
|
Date |
Therapy |
Cell number |
|
2017/09/27 |
CD19 CART |
9*10^7 |
|
2018/04/24 |
CD19 CART |
1.30*10^8 |
|
PSMA CART |
1.23*10^8 |
4SCAR (CD19, PSMA) after lymphodepletion with Flu-Cy
CRS Grade: 1 ; other side effects: no
Treatment response: PR Quality of Life: improved
Progression-free interval: 1 years 10 months
Current status: Alive with disease
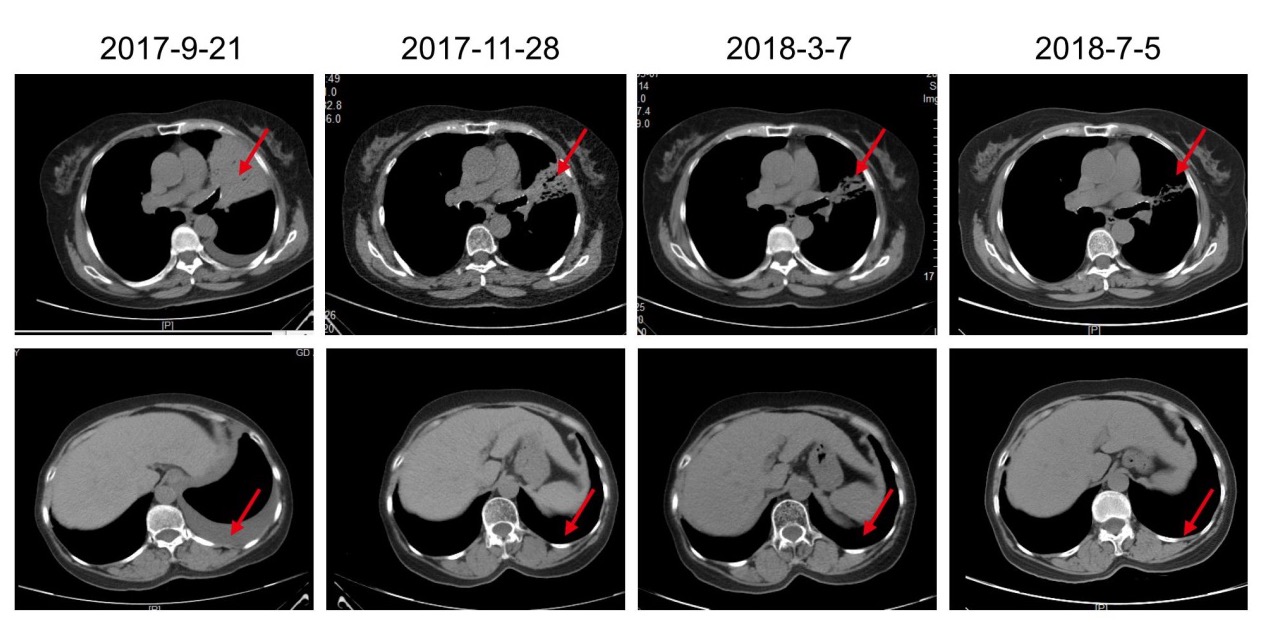
Disease images before and after CART treatment:
References:
1)Chang LJ, Dong LJ, Zhu J. et al. (2015) 4SCAR19 Chimeric Antigen Receptor-Modified T Cells As a Breakthrough Therapy for Highly Chemotherapy-Resistant Late-Stage B Cell Lymphoma Patients with Bulky Tumor Mass. Blood 126 (23), 264.
2)Jing HM, Chang LJ, Chen MY, Bao F, Wang J, Wang WM, Liu YY, Kuo HH, Liu YC, Dong LJ. (2015) Up-Regulation of CAR-T Cells after G-CSF and Dexamethasone Treatment in a Defuse Large B Cell Lymphoma Patient Receiving 4SCAR19 T Cell Therapy. Blood 126 (23), 5130.
3)L-J Chang, Lujia D, Liu Y-C, Tsao S-T, Li Y-C, Liu L, Gao Z, Tan X, Lu D-P, Zhang J-P, Wang J-B, Ying Y-M, Zhang L-P, Zheng H, Wang K, Zheng X-L, Wang H-X, Lai X, and Li D (2016) Safety and Efficacy Evaluation of 4SCAR19 Chimeric Antigen Receptor-Modified T Cells Targeting B Cell Acute Lymphoblastic Leukemia - Three-Year Follow-up of a Multicenter Phase I/II Study. Blood 128 (22), 587.
4) Hao L, Li T, Chang L-J and Chen X. (2017) Adoptive immunotherapy for B-cell malignancies using CD19-targeted chimeric antigen receptor T cells: a systematic review of efficacy and safety. Curr Med Chem.
5)Chang, L.-J., Li, Y. Tu, S. et al. Phase I/II Trial of Multi-Target Chimeric Antigen Receptor Modified T Cells (4SCAR2.0) Against Relapsed or Refractory Lymphomas. (2018) Blood 132:225.